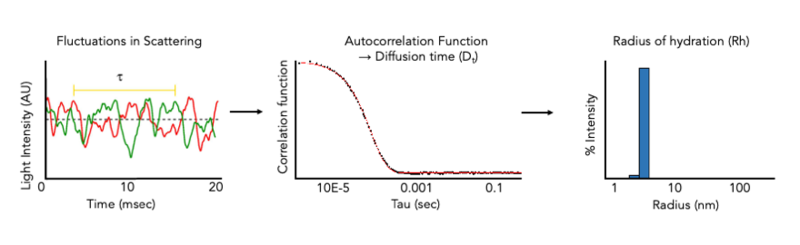
Dynamic Light Scattering (DLS)
DLS Instrumentation
DLS at the CMI
The CMI has Wyatt DynaPro Plate Reader III. The DynaPro Plate Reader III measures dynamic light scattering (DLS) and Static Light Scattering (SLS) at high-throughput on many sample types in standard microwell plates. and can be used to rapidly assess protein quality in solution. DLS measurements of hydrodynamic radius allow the monodispersity and aggregation state of a sample in solution to be measured in minutes and can be used to monitor protein quality during any (or all) stages of a protein purification project, or after storage. DLS can also be used to size a variety of nanoparticles, including liposomes, micelles, bicelles, and nanodiscs and for aggegation analysis of chemical compounds.

Wyatt DynaPro Plate Reader III Applications and Features
Applications
- Rh and Mw of a wide range of particles (proteins, liposomes, nanoparticles and other macromolecules)
- Monodispersity for rapid protein quality test
- Protein stability from thermal denaturation
- Aggregation analysis of samples, including chemical compounds
-
Multipeak Resolution
- The peak resolution limit in dynamic light scattering is 5X in size.
-
Highly unlikely to resolve oligomers, such as dimers and trimers, from the monomer
- Mixtures appear as a single broad peak
- High-order oligomerization can be detected (>5-10mer)
Key Features
- Measure Rh from 0.5 nm to 1000 nm
- Measure Mw from 1 to 1000 kDa
- Sample volume from 4 μL to 150 μL
- Sensitivity for size down to 12.5 μg/mL IgG
- Measure in 96-1536 well microplates
- Temperature ramps from 4°C to 85°C
Data Files - About CMI Data Files
Users are responsible for storage of all raw and processed data collected at the CMI.
- Users should have a plan to copy or transfer all raw and process data to their own local or cloud storage system.
- While the CMI allows temporary local storage of CMI User data on the instrument computer, we make no guarantees on the security or long-term availability of any data at the CMI.
- For most (but not all) CMI technologies, the raw data files and recommended readable exports are relatively small and can be readily transferred electronically.
- See specific instruments for exceptions and for details about the software, data file types and recommended data exports.
Data Sharing:
- Currently, a Generalist Repository is the recommended data repository for most CMI data types, as stable specialist data repositories have not been established.
Data Files - DLS - DynaPro Plate Reader III
| Technology | Dynamic Light Scattering (DLS) | ||
| Instrument | Wyatt Dynapro Plate Reader III | ||
| Recommended Repository | Generalist Repository | ||
| Software Type | Data Collection & Analysis | ||
| Current Version | Dynamics, Version 7.10.1.21 | ||
| Data Files (Type, ~size) | experiment file | .dexp | ~500KB/measurement |
| Readable Exports | Autocorrelation functions (all) | .csv | 20-100 KB/experiment |
| Autocorrelation functions (indiv) | .csv | 10 KB/measurement | |
| Autocorrelation errors (indiv) | .csv | 10 KB/measurement | |
| individual acquisitions | .csv | 30-50 KB/measurement | |
| Well image | .jpeg | 350 KB/measurement | |
| Regularization fit (all) | .csv | 10 KB/measurement | |
| Regularization fit (indiv) | .csv | 10 KB/measurement | |
| peaks tables | .csv | 10 KB/experiment | |
| results tables | .csv | 5 KB/experiment | |
Light Scattering Data Collection Services
Light Scattering Services Description
In addition to instrument training, the CMI is now offering basic Light Scattering Data Collection services, including:
Static Light Scattering: SEC-MALS or Mass Photometry
- mass determination in solution
- oligomeric state
Dynamic Light Scattering
- hydrodynamic radius
- buffer optimization
Mass Photometry Service Overview
Mass Photometry
Standard Mass Photometry Service
For proteins and protein complexes at least 30 KDa, without high molecular weight modifiers (modifiers of mass >5% total).
- Detector calibration with your buffer using a protein standard
-
Basic Data Analysis, in triplicate,
- Mass for each major peak
- Population distribution of major peaks
AAV Mass Photometry Services
For AAVs (replication-incompetent, ~3-5 MDa). Requires Empty Capsid control, provided by the user.
Empty/Full Service
- Detector calibration with your buffer using a protein standard
-
Basic AAV Analysis, in triplicate,
- Empty/Full population distribution
AAV Extended Service
- Detector calibration with your buffer using a protein standard and a DNA standard on poly-lysine coated slides
-
Extended AAV Analysis, in triplicate,
- Empty/Full population distribution
- Mass calculated for DNA cargo
SEC-MALS Service Overview
Size-exclusion Chromatography with Multi-Angle Light Scattering (SEC-MALS)
CMI SEC-MALS System: Wyatt Dawn Heleos with in-line DLS, RI UV, Agilent chromatography
Standard SEC-MALS Service
for proteins and protein complexes between 15 KDa and 1 MDa. Prior purification by size-exclusion chromatography (SEC) is strongly recommended.
Includes:
- Column equilibration with your buffer (pH at or below pH 7.5) or with PBS
- BSA Standard to test column performance and normalize detectors
- SEC separation of your protein sample(s)
-
Basic Data analysis, including:
- Molar Mass for each major peak
- Hydrodynamic Radius from DLS (when light scattering signal is sufficient)
Additional Services
-
Protein Conjugate Analysis
- required for samples with high molecular weight modifiers, such as glycosylation or detergent micelle (modifiers of mass >5% total)
- requires modifier dn/dc
- dn/dc measurements of detergent or protein samples
DLS Service Overview
Dynamic Light Scattering (DLS)
CMI DLS Instrument: Wyatt Dyanpro Plate Reader III
Standard DLS Service
For proteins and biopolymers up to 1000 nm in diameter. Includes:
- Cumulants and Regularization fit of Hydrodynamic Radius (Rh), 1 nm - 1000 nm
- Polydispersity Analysis
Additional Services
- Aggregation Analysis - Small molecule
- Buffer optimization
Data Collection Fees Summary
Data Collection
- Limited Data Collection Services are offered.
- Service fees are based on labor and supplies costs, and will be charged for all completed services, regardless of experimental outcome.
-
Before submitting samples for data collection, users must approve the estimated charges and be given a date and time for sample delivery.
- External Users will also be required to submit a PO and a signed CMI User Agreement.
-
Most CMI Data Collection Services include a setup fee plus a per-sample data collection fee.
- Some services include replicate measurements by default in the per-sample fee. For others, there is a reduced-price replicate measurement fee, if collected in the same dataset.
- Nanobody services not available to commercial users at this time.
- Current Harvard Life Lab commercial users are offered a 25% discount off the standard commercial rates.
DLS Resources
CMI DynaPro DLS Getting Started Guide, guide to DynaPro Plate Reader III.
CMI Dynapro SLS Getting Started Guide, guide to DLS with static light scattering measurements.
Tips for evaluating correlation function, adapted from DYNAMICS User’s Guide (M1406 Rev. F).
DLS Technology page from Wyatt Technologies.
DLS Supplies
0.02 μm filters for sample preparation, eg. Whatman Anatop 10, 0.02 μm, 10 mm inner diameter, Part Number 6809-1002
Clear-bottom Black Microplates:
| number of wells | 384 | 384 | 96 |
| Manufacturer | Aurora Microplates | Greiner Bio-One | Greiner Bio-One |
| Part Number | ABM2‐10100A | 781 892 | 655 892 |
| base material | 188 μm film | glass | glass |
| Minumum Volume | 25 μl | 50 μl | 100 μl |
| Temperature Range | 20-85 C | 20-37 C | 20-37 C |
| SLS Compatible | yes | yes | yes |
| Recommended For |
high temperature/ thermal stability |
Room Temp | Room Temp |
Assay Buffers
- DLS is compatible with a range of buffers
- Use filtered buffers
-
Measure scattering of buffer control
- Detergents frequently have micelle size similar to that of proteins and scatter significantly
Samples
-
DLS is very sensitive to small amounts of aggregates.
- Significant aggregates will make accurate size measurements difficult.
- Samples should be filtered or centrifuged (6000g, 10-30 min) prior to transfer to the clear bottom plate to remove large aggregates and precipitates.
-
The minimum amount of sample required depends on the size of the molecule, as large molecules scatter more light than small molecules.
- For most protein samples, concentrations of 0.2 mg/ml or higher are recommended.
- As little as 0.125 mg/ml of lysozyme or 12.5 μg/ml of IgG can be detected.
-
Small molecule aggregation analysis should be done at working concentrations in and working solvents (e.g. 5% DMSO).
- Solvent controls are essential. DMSO itself is prone to aggregation.
-
When working with membrane proteins, note that empty detergent micelles will not resolve from protein-filled micelles (except when empty micelle varies in size by 5-10X)
- Avoid excess detergent by purifying membrane proteins by affinity and/or size-exclusion chromatography and avoid concentrating after purification
