Differential Scanning Fluorimetry (DSF)
DSF at the CMI
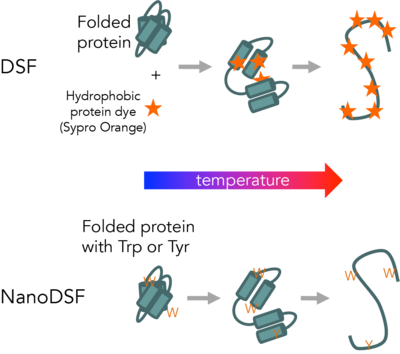
Differential Scanning Fluorimetry (DSF) measures protein unfolding by monitory changes in fluorescence as a function of temperature. Conventional DSF uses a hydrophobic fluorescent dye that binds to proteins as they unfold. NanoDSF measures changes in intrinsic protein fluorescence as proteins unfold.
The CMI has a modified Life Technologies Quant Studio 6/7, for conventional DSF. The CMI has the Life Technologies Protein Thermal Shift Analysis software for DSF data fitting.
The CMI has a Prometheus NT.Plex instrument from NanoTemper Technologies with aggregation optics. The CMI has these Data collection and analysis software packages: PR.ThermControl for thermal stability data collection, PR.ChemControl for chemical stability data collection, PR.TimeControl for time interval data collection, and PR.Stability Analysis for advanced data analysis.
DSF (Conventional DSF, Protein Thermal Shift Analysis)
NanoDSF
NanoDSF is a modified differential scanning fluorimetry method which monitors intrinsic tryptophan and tyrosine fluorescence as a function of temperature, time, or denaturant concentration. Tryptophan and tyrosine fluorescence intensity and wavelength maximum will vary as the local chemical environment changes, with significant changes occurring as buried or packed aromatic side chains become solvent exposed upon unfolding. NanoDSF measures fluorescence intensity at 350 nm and 330 nm and compares the ratio as a function of temperature or denaturant concentration. NanoDSF can be used for a broader range of protein samples than traditional DSF and has significantly higher throughput and lower sample consumption than DSC or CD. Free energies of folding and temperatures of unfolding measured using NanoDSF are comparable to values determined by DSC for a range of sample types. The only significant limitation is that the protein of interest must contain aromatic amino acids (tryptophan or tyrosine).
Data Files - About CMI Data Files
Users are responsible for storage of all raw and processed data collected at the CMI.
- Users should have a plan to copy or transfer all raw and process data to their own local or cloud storage system.
- While the CMI allows temporary local storage of CMI User data on the instrument computer, we make no guarantees on the security or long-term availability of any data at the CMI.
- For most (but not all) CMI technologies, the raw data files and recommended readable exports are relatively small and can be readily transferred electronically.
- See specific instruments for exceptions and for details about the software, data file types and recommended data exports.
Data Sharing:
- Currently, a Generalist Repository is the recommended data repository for most CMI data types, as stable specialist data repositories have not been established.
Data Files - DSF - QuantStudio 6/7
| Technology | Differential Scanning Fluorimetry (DSF) | ||
| Instrument | Life Technologies Quant Studio 6/7 | ||
| Recommended Repository | Generalist Repository | ||
| Software Type | Data Collection | ||
| Current Version | QS Real-Time PCR Software, version 1.7.1 | ||
| Data Files (Type, ~size) | experiment file | .eds | 2-10 MB/plate |
| Software Type | Data Analysis | ||
| Current Version | Applied Biosystems Protein Thermal Shift, version 1.2 | ||
| Data Files (Type, ~size) | experiment file | .eds | 2-10 MB/plate |
| Readable Exports | raw data | .csv | 2 MB/plate |
| analyzed data | .csv | 12 KB/project | |
| analyzed data | .txt | 29 KB/project | |
Data Files - DSF - Prometheus
| Technology | Nano Differential Scanning Fluorimetry (nanoDSF) | ||
| Instrument | NanoTemper Prometheus NT.Plex | ||
| Recommended Repository | Generalist Repository | ||
| Software Type | Data Collection (Thermal Denaturation) | ||
| Current Version | PR.ThermControl, Version 2.3.1 | ||
| Data Files (Type, ~size) | experiment file | .prc | 10-30 MB/project |
| raw data | .xslx | 2 MB/project | |
| Software Type | Data Analysis | ||
| Current Version | PR.Stability Analysis, Version 1.1 | ||
| Data Files (Type, ~size) | analysis file | .pra | 2-6 MB/project |
| Readable Exports | processed data | .xslx | ~500 KB/sample |
| results table | .xslx | ~30 KB/project | |
| Software Type | Data Collection (Chemical Denaturation) | ||
| Current Version | PR.ChemControl, Version 1.4.3 | ||
| Data Files (Type, ~size) | experiment file | .prcc | 10 MB/project |
| Readable Exports | raw data | .xslx | 6 KB/sample |
| Software Type | Data Collection (Time Control) | ||
| Current Version | PR.TimeControl, Version 1.0.2 | ||
| Data Files (Type, ~size) | experiment file | .prtime | 2 MB/project |
| Readable Exports | raw data | .xslx | 60 KB/project |
DSF Data Collection Services
DSF Service Overview
In addition to instrument training, the CMI is now offering basic protein Differential Scanning Fluorimetry services, including protein thermal shift analysis and buffer optimization.
Differential Scanning Fluorimetry (DSF) with fluorescent dye or using intrinsic protein fluorescence
Life Technologies Quant Studio 6/7
- Conventional DSF, protein thermal stability using hydrophobic dye Sypro Orange
- Buffer optimization
NanoTemper Technologies Prometheus NT.Plex
- NanoDSF, protein thermal stability using intrinsic protein fluorescence
- Chemical denaturation
- Buffer optimization
Data Collection Fees Summary
Data Collection
- Limited Data Collection Services are offered.
- Service fees are based on labor and supplies costs, and will be charged for all completed services, regardless of experimental outcome.
-
Before submitting samples for data collection, users must approve the estimated charges and be given a date and time for sample delivery.
- External Users will also be required to submit a PO and a signed CMI User Agreement.
-
Most CMI Data Collection Services include a setup fee plus a per-sample data collection fee.
- Some services include replicate measurements by default in the per-sample fee. For others, there is a reduced-price replicate measurement fee, if collected in the same dataset.
- Nanobody services not available to commercial users at this time.
- Current Harvard Life Lab commercial users are offered a 25% discount off the standard commercial rates.
CMI QuantStudio DSF Getting Started Guide
Protein Thermal Shift Studies manual from Applied Biosystems by Life Technologies
All Experiments:
- 96-well FAST-block optical plate, eg.: LifeTechnologies MicroAmp FAST optical 96-well reaction plate, 0.1 ml, 4346907
- optical adhesive film, eg.: LifeTechnologies MicroAmp Optical Adhesive Film, 4360954
DSF/Protein Thermal Shift Experiments
- DSF compatible dye, eg.: LifeTechnologies Protein Thermal Shift Dye Kit, 4461146 (Sypro Orange)
- samples, ligands, buffers
qPCR Experiments
- qPCR reagents (eg. LifeTechnologies PowerUp SYBR Green Master Mix, A25742)
- primers and templates
qPCR Resources
CMI QuantStudio qPCR Getting Started Guide
Quant Studio 6/7 Quick Reference Guide from Applied Biosystems by Life Technologies
NanoDSF Resources
NanoDSF Supplies
-
NT.Plex Capillary Chips
- 2x 8 Standard 24-Capillary Chips, NanoTemper Catalog # PR-AC002
-
2x 8 High Sensitivity 24-Capillary Chips, NanoTemper Catalog # PR-AC006
- 384-well plates for loading capillaries
- Protein samples, ligands, buffers
